Mesenchymnal Stem Cells and uses in veterinary medicine


Mesenchymal Stem Cells

MSCs are undifferentiated cells that have the function of maintaining tissue or organs throughout life. These cells are present in various tissues and organs of the adult and, in the presence of a tissue injury, they are naturally recruited to assist in their recovery. However, over the years, the niches that produce them, gradually become weaker, which impairs the natural process of regeneration.
Read More
In this context, the biotechnology of extracting and cultivating these cells in the laboratory enabled the enhancement of this naturally existing system. Stem Cells, when applied, are attracted to the injury and contribute to the restoration of the injured tissue, differentiating themselves (when possible) in healthy cells of the compromised organ and concomitantly exercising a paracrine action, which is mediated by the microenvironment in which they were attracted. Stem Cells are able to perceive through the local cell signals, what are the needs of that injured tissue and then produce countless and diverse active biomolecules such as interleukins, growth factors and others, which, finally, promote important actions in the recovery of the injury, as angiogenic, anti-fibrotic, anti-apoptotic, anti-inflammatory and immunosuppressive action, in addition to positively stimulating Stem Cells residing in the microenvironment itself.
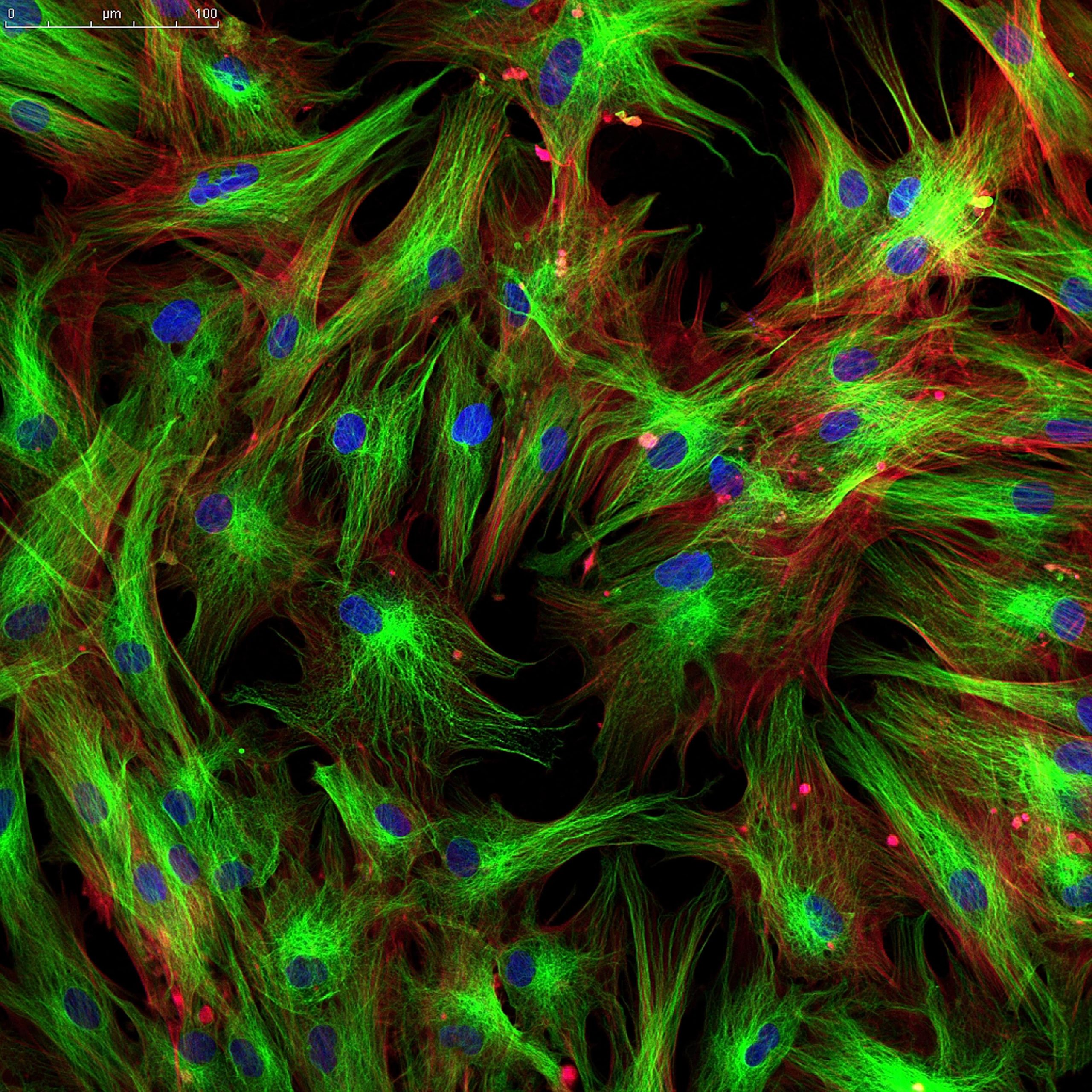
Immunomodulatory and Anti-inflammatory action of MSCs

In order to understand the immunomodulatory mechanism of MSCs, a basic understanding of the components involved in these actions is necessary. In general, regardless of the underlying etiology, in all the different pathologies, there is a response from the organism to eliminate the causative agent, and or try to repair the damage done by it. For these actions, innate cells (neutrophils, basophils, eosinophils, macrophages, dendritic cells, B lymphocytes, T lymphocytes and NK cells) are involved, which, depending on the profile of the etiologic agent, will act in different ways. Cytokines are produced by innate cells and various types of cells at the site of the lesion, being responsible for mediating anti-inflammatory, immunosuppressive reactions, or for regulating the allergenic response, in an attempt to balance the diseased micro-environment and try to restore its function. When this mechanism is inefficient or when there is an exaggerated production of pro-inflammatory cytokines from the lesion, it can manifest itself systemically with hemodynamic instability or metabolic disorders. After serious injuries or infections, the exacerbated and persistent response of Th1 cytokines can contribute to target organ damage, leading to multiple organ failure and death.

Read More
Mesenchymal stem cells (MSCs) use several molecular mechanisms to suppress innate immune cells, being able to intelligently orchestrate this entire immune system, meaning, they are able to sense the needs of the diseased microenvironment and react to it, balancing and consequently helping in tissue recovery. The MSCs do this action by secreting several soluble factors such as: IL-4, IL-6, IL-1, TGF-β, HGF, IDO and PGE2 (NAGAYA et al., 2005; CAPLAN, 2006), being this the central mediator of the immunomodulatory response on the other cells of the immune system. Next, we will describe how the physiological mechanisms of MSC immunomodulation work.
The anti-inflammatory actions that MSCs have, are wide and significant. Inflammation is triggered by the production of pro-inflammatory cytokines by the defense cells. An example are macrophages, which depending on their form of activation can exert a pro-inflammatory action (M1 macrophage), as they produce pro-inflammatory cytokines, nitric oxide (NO), providing tissue damage; or anti-inflammatory (macrophage M2) related to tissue repair, because they produce essential mediators in the resolution of inflammation, tissue remodeling and in the elimination of tissue debris, facilitating the survival and proliferation of resident cells and promoting the repair of wounds and adequate healing ( SHALHOUB et al., 2011). In this context, the MSCs when secreting interleukin-4 (IL-4) and IL-13, condition the favoring of the expression of the class 2 (M2) macrophage, which is characterized by the production of anti-inflammatory interleukin (IL-10), increased CD206, decreased expression of IL-12, tumor necrosis factor-α (TNF-a) and NO (KIM et al., 2009; SPAGGIARI et al., 2013; CHO et al., 2014). MSCs antagonize the expression of the M1 phenotype, reduce the expression of CD86 and MHCII in macrophages, thus decreasing their stimulatory power (MAGGINI et al., 2010). In addition to macrophages, neutrophils are important phagocytes of the innate immune system. In response to the detection of microbial molecules, neutrophils produce a large amount of oxidative microbicidal products in the so-called oxidative respiratory explosion (WITKO et al., 2000). Respiratory explosions are also closely associated with neutrophil apoptosis (WATSON et al., 2002). MSCs inhibit apoptosis of neutrophils, even under conditions of IL-8-mediated activation, via IL-6 derived from MSCs (RAFFAGHELLO et al., 2008; MAQBOLL et al., 2011). It is thought that MSCs can apply this effect to preserve the pool of undivided neutrophils found in bone marrow sinusoids. MSCs also prevent respiratory bursts of neutrophils, an effect that aligns with MSM immunosuppression, but has no effect on neutrophil phagocytosis, matrix adherence or chemotaxis (RAFFAGHELLO et al., 2008).
Mast cells strongly contribute to allergic responses, especially through the release of pro-inflammatory cytokines and granules containing histamine. MSCs suppress the ability of mast cells to degranulate and produce TNF-α (BROWN et al., 2011). In a model of passive cutaneous anaphylaxis in vivo, MSCs reduced inflammation promoted by mast cells. In these experiments, MSCs-mediated immunosuppression was dependent on the positive regulation of cyclooxygenase-2 in MSCs and their production of PGE2, which suppressed mast cells via EP4 receptor binding.Natural killer cells (NK) are innate immune cells that, in addition to producing pro-inflammatory cytokines, are cytotoxic in relation to intracellular cells infected with pathogens and cancer cells. The cytotoxicity of NK cells is regulated by inhibitory and activating receptors, in addition to the target cell’s MHC expression levels and antibody-dependent cell cytotoxicity. An example of this is the expression of indolamine-2,3-dioxigenase by MSCs capable of inhibiting the activation and proliferation of NK cells (SPAGGIARI et al., 2006), of reducing the expression of NK activating receptors, including 2B4 and NKG2D ( SOTIROPOULOU et al., 2006) and to reduce the production of the cytokines secreted by them. In addition, the recently isolated NKs were not cytotoxic to MSCs, but acquired cytotoxicity after 4 days of cultures with IL-15. The neutralization of PGE2 and the transforming growth factor-β (TGF-b), both thought to contribute to the immunosuppression of MSCs, surpass the MSC-mediated suppression of NK proliferation.
Dendritic cells (DC) unite the innate and adaptive immune systems, as they function both as producers of cytokines and also as potent antigen presenters. The DCs absorb the antigen and during maturation and activation, increase the expression of costimulatory molecules (CD40, CD80, CD83 and CD86), migrate to secondary lymphoid organs and present antigen to the T cells (primary adaptive immune response). During the initiation of T cells, the DCs also produce a mixture of cytokines that affect the evaluating function of T cells. MSCs affect most of these processes: they inhibit endocytosis by the DCs, increase the regulation of MHC, CD40, CD80, CD83 and CD86 during differentiation and prevent further increase in CD40, CD83 and CD86 expression during maturation (NAUTA et al., 2006). MSCs also interfere with the ability of DC to produce IL-12 and activate allogeneic T cells (ZHANGW et al., 2004; NAUTA et al., 2006). In addition, CTM blocks the generation of dermal CDs from precursors of CD14 + CD1a derived from CD34 and those derived from immature monocytes (NAUTA et al., 2006). Monocytes cultured under conditions of CD differentiation in the presence of MSCs, do not proliferate and remain in the G0 state (RAMASAMY et al., 2007). One study revealed that treatment with MSC inhibited CD maturation in vivo, as well as cytokine secretion and migration to lymphnodes (CHIESA et al., 2011), resulting in insufficient T-cell initiation in the ganglia lymphatic. MSCs mediate modulation of DCs. For example, through bone marrow progenitors (DJOUAD et al., 2007) and PGE2 secreted by MSCs, there is an inhibition of CD differentiation and a decrease in the conversion of mature DCs (CD11c + B220-CD), into a regulatory subset (ZHANG et al., 2014).
The cells of the adaptive immune system, particularly the B and T lymphocytes, are composed of billions of unique clones that, unlike other innate immune cells, recognize highly specific molecules (usually peptides). Each clone expands the recognition of the antigen and reaches an effective state to eliminate the pathogen present. B cells are specialized in the production of antibodies, which play multiple roles in the direct neutralization of pathogens, promoting opsonization to neutralize phagocytic ingestion and activation of other immune cells. Naive B cells are activated by binding to the B cell receptor (BCR), binding to CD40 / CD40L and binding to Toll-like receptors (TLR) of microbial products (FRANQUESA et al., 2012). In response to activation, B cells proliferate and differentiate into plasma cells, which produce antibodies. MSCs inhibit the proliferation of B cells in vivo and in vitro, by arrest at the G0 / G1 check point, without inducing apoptosis (FRANQUESA et al., 2012; TABERA et al., 2008). In addition, MSCs reduce the production of IgG, IgM and IgA during in vitro co-culture with B cells and suppress the expression of the chemokine receptor in B cells (CORCIONE et al., 2006). In in vivo transplants, MSC injections led to a reduction in allo-specific antibodies and promoted long-term graft acceptance (GE et al., 2009; FRANQUESA et al., 2012). In a form of experimental autoimmune encephalomyelitis (EAE) mediated by proteolipid protein (PLP) and in a murine form of multiple sclerosis (RAFEI et al., 2009), mice that received MSCs exhibited an inhibition of PLP-specific antibodies (GERDONI et al. , 2007). This is due to cell-cell contact and the soluble factors synthesized by MSCs that suppress B cell function.T cells of adaptive immune systems are divided into CD4 + and CD8 + lines, which can be sub-grouped into different effector subsets. After activation via single T cell receptors (TCR) and costimulation by antigen presenting cells (APCs), such as DC, T cells proliferate rapidly and differentiate into effector cells. CD4 + effector T cells develop as IFNg-producing TH1 cells, TH2 cells are producers of IL-4 and IL-13, Treg cells are producers of IL-10 and TH17 are producers of IL-17. CD8 + T cells are mainly considered as cytotoxic T lymphocytes and produce cytotoxic granules that kill infected and cancer cells; however, they can differentiate into other subtypes such as their CD4 + T cell counterparts.MSCs inhibit the proliferation of all T cells, except T reg cells, by arrest in the G0 / G1 cell cycle phase (DI NICOLA et al., 2002; ZAPPIA et al., 2005). This inhibition is also independent of MHC, since autologous and allogeneic MSCs have this same anti-proliferative effect. This T cell suppression mechanism occurs because MSCs by regulating the inducible nitric oxide (iNOS) synthesis, which produces NO (REN G et al., 2008). Another important event is that MSCs modulate the production of cytokines released by T cells, through the production of immunomodulatory molecules, such as hepatocyte growth factor (HGF), TGF-B and PGE2 (DI NICOLA et al., 2002), being able to suppress the production of IFNy from TH1 cells, they promote the secretion of IL-4 from TH2 cells and increase the proportion of T reg (AGGARWAL et al., 2005). In addition, MSCs can suppress cytotoxicity of cytotoxic T cells by a soluble factor (RASMUSSON et al., 2003). When administered viral peptides and tumor antigens, MSCs suppress the death of cytotoxic T cells and are not recognized as targets of infection or foreign cells, despite the expression of MHC-I (RASMUSSON et al., 2007; UCCELLI et al., 2008 ; MORANDI et al., 2008).In vivo, MSCs have been used extensively in preclinical experimental disease environments involving pathogenic T cells. Some of the first reports show improvement mediated by MSC in cases of peptide-induced EAE (MOG – myelin oligodendrocyte glycoprotein), which induces a neuroinflammatory disease mediated by TH1 and TH17 cells (ZAPPIA et al., 2005; RAFEI et al., 2009). In this context, the polarization of these cells was inhibited in vivo, and HGF derived from MSCs suppressed EAE at the same time that it promoted a beneficial neurotropic effect (ZAPPIA et al., 2005; RAFEI et al., 2009; BAI et al. , 2012). In one study, MSCs suppressed skin graft rejection in monkeys, which was associated with suppression of T cell proliferation (BARTHOLOMEW et al., 2002). In a model of streptokine-induced autoimmune diabetes, MSCs inhibited the T-cell-mediated destruction of insulin-secreting β cells in the pancreas (URBAN et al., 2008). MSCs also suppressed the proliferation of auto-reactive T cells in collagen-induced arthritis, in addition to decreasing TNF-α production and supporting the generation of T reg cells (AUGELLO et al., 2007). These studies demonstrate the immense potential of using MSCs as immunomodulators.In summary we can say:Anti-inflammatory action: MSCs promote an anti-inflammatory action because they significantly suppress the production of pro-inflammatory cytokines produced by macrophages and dendritic cells, favorably stimulate the polarization of class 2 (M2) macrophages, which have anti-inflammatory action. inflammatory, decrease monocyte differentiation in dendritic cells and secrete anti-inflammatory cytokines such as IL10.Immunosuppressive action: MSCs suppress the immune system because they decrease the production of T lymphocytes, production and cytotoxicity of cytotoxic T lymphocytes and NK cells, to the detriment of T reg lymphocytes that are stimulated and also decrease the proliferation and differentiation of B lymphocytes into plasma cells.Allergogenic immunomodulatory action: MSCs significantly decrease the allergenic response, as they suppress the ability of mast cells to degranulate and produce TNF-α.
Pharmacodynamics of Mesenchymal Stem Cells (MSCs)

Chemotaxis of MSCs to Injured tissue
When MSCs are transplanted into the organism, are recruited bythe lesion when they release chemotactic signs and increase the expression of specific adhesion molecules. This mechanisms are possible because they express adhesion molecules (selectins, integrins a4 / B1 and CD44) that connect to the sites of connection of vascular cell adhesion molecule 1 (VCAM-1), allowing the attachment of these cells to blood vessels and later endothelial transmigration. Circulating MSCs in blood vessels trespassing the vascular wall, coming into direct contact with the tissue cells. What defines the strength of this chemotaxis is the inflammatory condition of the injured tissue.
Damaged cells sustitution
Exogenous MSCs are able to differentiate themselves into specific cell types through Cellular Differentiation, Cellular Trans differentiation and Cellular Fusions. Furthermore, these cells in contact with the microenvironment stimulate intrinsic MSCs to help repair the tissue using this same mechanism
Cellular differentiation
Differentiation is a process in which undifferentiated stem cells derive into specialized and functional cells. This phenomenon occurs by biochemical stimuli both in vivo and in vitro. These stimuli can naturally occur in the organism in vivo, through the secretion of growth factors produced by the intrinsic cells of the tissue, but in vitro the stimuli occur artificially using supplements and conditioning of specific culture media for each selected cell type.
Cellular Trans differentiation
The MSCs extracted from adipose tissue have mesodermal origin, however, are capable of being transdifferentiated into cells of other embryonic germ layers (endodermal and ectodermal), for example, into neuronal or epithelial cells that are of ectodermal origin.
Cellular fusion
The MSCs introduced into the organism, fuses with host cells and improves the cell function.
Cellular recruitment
MSCs transplanted into the organism encourages intrinsic MSC recruitment and other cell types from other niches to assist in the repair of the tissue.
Anti-inflammatory effect
The MSCs has extensive and significant actions in inflammatory conditions. These cells negatively regulate the production of pro-inflammatory cytokines from organism defense cells and secret interleukins with anti-inflammatory action, such as IL-4, IL-10 and IL-13 interleukins. The MSCs secrete IL-4 and IL-13, which conditions the expression of the macrophage of class 2 (M2), which is characterized by the production of anti-inflammatory interleukin (IL-10), increased CD 206, expression of IL-12 expression, tumor necrosis factor-α (TNF-a) and NO 9, 10, 11 and antagonizing the expression of the M1 phenotype, decreasing the expression of CD86 and MHCII in macrophages and therefore their stimulatory power. The MSCs inhibit the apoptosis of neutrophils, even under activation conditions mediated by IL-8, via IL-6 derived from MSCs.The MSCs provides a significant control of mast cells, which strongly contribute to allergic responses, both by releasing pro-inflammatory cytokines and by releasing granules containing histamine. In this situation, MSCs suppress mast cell degranulation and consequently TNF-α production
Immunosuppressive effect
MSCs inhibit the proliferation and cytotoxicity of all T cells, with the exception of T reg cells that secrete cytokines with anti-inflammatory action (IL-10). This mechanism, which is multifactorial, occurs because MSCs regulate the synthesis of induced nitric oxide (iNOS), promote the imprisonment of these cells in the G0 / G1 cell cycle phase, and modulate the production of cytokines released by T cells through the production of molecules such as hepatocyte growth factor (HGF), TGF-B and PGE2, which, in turn, are able to suppress IFNy production from TH1 cells, promote the secretion of IL-4 a from TH2 cells and thus increase the proportion of T reg. MSCs can inhibit the proliferation and cytoxicity of natural killer cells (NK), reduce the expression of NK activating receptors, including 2B4 and NKG2D and reduce the production of ammoniating cytokines produced by them, all through the expression / synthesis of indolamine-2,3-dioxygenase. MSCs inhibit endocytosis of dendritic cells (CD), increase the regulation of MHC, CD40, CD80, CD83 and CD86 during differentiation and prevent further expression of CD40, CD83 and CD86 during maturation. Furthermore, they interfere with the ability of CDs to produce IL-12 and activate allogeneic T cells; they block the generation of dermal CDs from CD14 + CD1 precursors – derivatives of CD34 and derivatives of immature monocytes. The prostaglandin E2 (PGE2) secreted by the CTM regulates the production and modulation of the CDs, in the latter, reducing the conversion of immature to mature CDs.
Antifibrotic Effect
MSCs secrete the liver growth factor that prevents and modulates the formation of tissue fibrosis. Faced with tissue injury, fibroblasts and the body’s defense cells secrete FGF, which activates the MSCs to the HGF secretion, thus preventing tissue fibrosis. It was demonstrated in a study that in the absence of these two growth factors, there is a reduction in the proliferation of intrinsic MSCs, resulting in a demarcated fibrinogenesis. Determining, that the administration of MSCs can prevent fibrosis.
Angiogenic effect
MSCs secrete bioactive factors involved in the process of new vessel formation, such as VEGF, FGF, HGF-1, SDF-1, and angiopoietin. The production of these growth factors stimulates the proliferation and differentiation of endothelial cells and smooth muscle cells, promoting the development of collateral vessels and increased capillary density in ischemic tissues.
Anti-apoptotic effect
In a scenario where MSCs are administered to treat acute injury, the first expected effect is to reduce the extent of cell death. MSCs secrete several bioactive molecules that have anti-apoptotic action, including: HGF, G-CSF, IGF, VEGF, EGF and TGF-β. In addition, MSCs activate the mitosis of progenitors, intrinsic SCs, and healthy host cells by secreting biomolecules such as: SDF-1, SCF, G-CSF, and angiopoietin-1.
- Segers VF, Van Riet I, Andries LJ, Lemmens K, Demolder MJ, De Becker AJ, et al. Mesenchymal stem cell adhesion to cardiac microvascular endothelium: activators and mechanisms. Am J Physiol Heart Circ Physiol. 2006;4:1370-7.
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-84
- Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291:886-93.
- Ryu HH, Lim JH, Byeon YE, Park JR, Seo MS, Lee YWet al. Functional recovery and neural differentiation after transplantation of allogenic adipose derived stem cells in a canine model of acute spinal cord injury. J Vet Sci. 2009;4:273-84.
- Da Silva Meirelles, L, Chagastelles, P. C, Nardi, N. B. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-13.
- Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001;104:1046-52.
- Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076-84.
- Nagaya N et al. Transplantation of mesenchymal stem cells improves cardiac function in a rat model of dilated cardiomyopathy. Circulation. 2005;8:112.
- Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:70.
- Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Exp Hematol. 2009;37:1445-53.
- Spaggiari GM, Moretta L. Cellular and molecular interactions of mesenchymal stem cells in innate immunity. Immunol Cell Biol. 2013;91:27-31.
- Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252.
- Raffaghello L, Bianchi G, Bertolotto M, Montecucco F, Busca A, Dallegri F, et al. Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells. 2008;26:151-62.
- Brown JM, Nemeth K, Kushnir-Sukhov NM, Metcalfe DD, Mezey E. Bone marrow stromal cells inhibit mast cell function via a COX2-dependent mechanism. Clin Exp Allergy 2011;41:526-34.
- Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-50.
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002;99:3838-43.
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood 2005;106:1755-61.
- Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815-22.
- Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. Stem Cells. 2006;24:74-85.
- Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood 2006;107:1484-90.
- Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-7.
- Zhang Y, Cai W, Huang Q, Gu Y, Shi Y, Huang J, et al. Mesenchymal stem cells alleviate bacteria-induced liver injury in mice by inducing regulatory dendritic cells. Hepatology 2014;59:671-82.
- Suga H, Eto H, Shigeura T, Inoue K, Aoi N, Kato H, et al. IFATS series: FGF-2- induced HGF secretion by adipose-derived stromal cells inhibits post-injury fibrogenesis through a JNK-dependent mechanism. Stem Cells. 2009 Jan;27(1):238-49.
- Li L, Zhang S, Zhang Y, Yu B, Xu Y, Guan Z. Paracrine action mediate the antifibrotic effect of transplanted mesenchymal stem cells in a rat model of global heart failure. Mol Biol Rep. 2009 Apr;36(4):725-31.
- Hung SC, et al. Angiogenic effects of human multipotent stromal cell conditioned medium activate the PI3K-Akt pathway in hypoxic endothelial cells to inhibit apoptosis, increase survival, and stimulate angiogenesis. Stem Cells. 2007;9:2363-70.
- Laflamme MA, Zbinden S, Epstein SE, Murry CE. Cell-based therapy for myocardial ischemia and infarction: pathophysiological mechanisms. Annual Review of Pathol. 2007;2:307-39.
- Tögel F et al. How cells change their phenotype. Nat Rev Mol Cell Biol. 2007;3:187-94
- Zhang W, Ge W, Li C, You S, Liao L, Han Q, et al. Effects of mesenchymal stem cells on differentiation, maturation, and function of human monocyte-derived dendritic cells. Stem Cells Dev. 2004;13:263-71.
Uses in veterinary medicine


Mesenchymal Stem Cells (MSC) in Acute and Chronic Kidney Disease

It is known that kidney disease is a chronic and progressive disease, that to date, there are no treatments to reverse this condition. Currently, there are numerous studies demonstrating the effectiveness of the use of MSC both in the acute and in the chronic phase. The mechanism used for this purpose is the paracrine and regenerative actions that these cells provide as soon as they come into contact with the injured tissue and its microenvironment. As mechanisms of action we can mention: Improvement in renal fibrosis, improvement in renovascular hypertension, improvement in the local inflammatory condition by immunomodulating the microenvironment, reducing the production of pro-inflammatory cytokines and increasing the production of anti-inflammatory cytokines, also acts promoting angiogenesis in ischemic situations. In addition, MSCs secrete hepatocyte growth factor (HGF), which is directly related to tubulogenesis. These actions delay the sequential loss of the nephrons and culminate in the improvement of renal function, observed by the reduction of azotemia and proteinuria with consequent improvement in the animals’ quality of life.
Read More
- Cavaglieri, R. C. et al. Mesenchymal stem cells delivered at the subcapsule of the kidney ameliorate renal disease in the rat remnant kidney model. Transplant Proc, v. 41, p. 947–951, 2009.
- Barros, M A et al. Immature Dental Pulp Stem Cells Showed Renotropic and Pericyte-Like Properties in Acute Renal Failure in Rats Cell Medicine, Vol. 7, pp. 95–108, 2015.
- Morigi M. et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol, v. 15, p. 1794–1804, 2004.
- Semedo P, et al. Mesenchymal stem cells ameliorate tissue damages triggered by renal ischemia and reperfusion injury. Transplant Proc. 2007;39(2):421-3.
- Morigi M et al The regenerative potential of stem cells in acute renal failure. Cell Transplant. 2006;15 Suppl 1:S111-7.
- Hiroshi Asanuma et al 2010. Arterially Delivered Mesenchymal Stem Cells Prevent Obstruction-Induced Renal Fibrosis.
- Oliveira-Sales EB et al. Mesenchymal Stem Cells (MSC) Prevented the Progression of Renovascular Hypertension, Improved Renal Function and Architecture. PLoS ONE 2013; 8(11): 78464.
- Gao X et al. Hepatocyte growth factor gene therapy retards the progression of chronic obstructive nephropathy. Kidney Int. 2002;62(4):1238-48.
- Semedo P et al. Early modulation of inflammation by mesenchymal stem cell after acute kidney injury. Immunopharmacol. 2009, 9:677-82.
- Quimby, J.M., Webb, T.L., Gibbons, D.S., Dow, S.W., 2011. Evaluation of intrarenal mesenchymal stem cell injection for treatment of chronic kidney disease in cats: A pilot study. Journal of Feline Medicine and Surgery 13, 418–426.
- Quimby, J.M., Webb, T.L., Habenicht, L.M., Dow, S.W., 2013. Safety and efficacy of intravenous infusion of allogeneic cryopreserved mesenchymal stem cells for treatment of chronic kidney disease in cats: Results of three sequential pilot studies. Stem Cell Research and Therapy 4, 48.
- Quimby, J.M., Webb, T.L., Randall, E., Marolf, A., Valdes-Martinez, A., Dow, S.W., 2015. Assessment of intravenous adipose-derived allogeneic mesenchymal stem cells for the treatment of feline chronic kidney disease: A randomized, placebo- controlled clinical trial in eight cats. Journal of Feline Medicine and Surgery.
- Semedo P et al. Immunosuppressive and remodelling properties of mesenchymal stem cells in a model of chronic kidney disease einstein. 2009; 7(4 Pt 1):469-79
- Cheng Chen and Jianquan Hou. Mesenchymal stem cell-based therapy in kidney transplantation Chen and Hou Stem Cell Research & Therapy (2016) 7:16.

Mesenchymal Stem Cells (MSC) in the Neurological Sequelae of Distemper

Distemper is a highly contagious viral disease and, depending on the course of the disease, can lead to neurological sequelae incompatible with life, due to its great demyelinating action. To date, there is no curative treatment, and only supporting therapies such as physiotherapy and acupuncture are possible. MSCs have been widely used in human diseases similar in character to distemper sequelae such as Multiple Sclerosis and Autoimmune Myeloencephalitis. MSCs are able to assist neurological regeneration both by transdifferentiating into neuronal cells and by stimulating the body’s own neural precursor cells. They are also capable of promoting the remyelination of oligodendrocytes, reducing the neural apoptotic action and immunomodulating the microenvironment, by decreasing the production of pro-inflammatory cytokines and increasing the production of anti-inflammatory molecules. These actions allow effective and permanent results.
Read More
- Riordan, N. H. et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. Journal of Translational Medicine. v. 7, p. 1479–5876, 2009.
- Chen L, et al. Human Neural Precursor Cells Promote Neurologic Recovery in a Viral Model of Multiple Sclerosis Stem Cell Reports J. 2014; Jun 3; 2:825-37.
- Uccelli A, et al. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011; Mar; 24(1):59-64.
- Jiang H, et al. Amelioration of experimental autoimmune encephalomyelitis through transplantation of placental derived mesenchymal stem cells Scientific Reports 2017; 41837.
- Barros M A. Avaliação do Transplante Alogênico de Células Tronco Mesenquimais isoladas de tecido adiposo em cães portadores de sequela neurológica causada pela Cinomose. Tese Doutorado apresentada na Universidade Federal de São Paulo – Unifesp – Escola Paulista de Medicina (2017).
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. 7. Ben-Hur T. Immunomodulation by neural stem cells. J Neurol Sci. 2008;265: 102–4.
- Brito, H.F.V.; Corat, M.A.F.; Santos, M.R.; Gilioli, R.; Passos, L.A.C.; Lancellotti, M.; Ferreira, F.; Min, L.L. Tratamento de Sequelas Neurológicas em cães, causadas por infecção pelo vírus da cinomose, através do transplante alogênico de células mononucleares de medula óssea. Cient. de Med. 2010; 8(24): 26-29.


Mesenchymal Stem Cells (MSC) in Osteoarthritis and Osteoarthrosis

Arthritis, also known as osteoarthritis or osteoarthrosis (OA), is a progressive and degenerative chronic joint disease, common in dogs, cats and horses. The available treatments are palliative in the quest to improve the animal’s quality of life. However, the frequent use of medications normally practiced (steroidal or non steroidal anti-inflammatory drugs), brings serious impairments over time. Alternatively, MSCs, due to their immunomodulatory characteristics, have the same anti-inflammatory effects as traditional medications, but without the side effects of these medications. In addition to the anti-inflammatory action, MSCs stimulate synoviocytes to produce more synovial fluid, promote cartilage regeneration by differentiating into chondrocytes and chondroblasts, and delay the OA progression through anti-apoptotic action. These actions bring a significant improvement in the animals’ quality of life in the long term.
Read More
- Shen-Yang Tsai, Yun-Ching Huang, Ling-Ling Chueh, Lih-Seng Yeh, Ching-Shwun Lin. Intra-articular transplantation of porcine adipose-derived stem cells for the treatment of canine osteoarthritis: A pilot study. World J Transplant. 2014; Sep 24;4(3):196-205.
- Black, L. L. et al. Effect of intraarticular injection of autologous adipose-derived mesenchymal stem and regenerative cells on clinical signs of chronic osteoarthritis of the elbow joint in dogs. Vet Ther, v. 9, p.192–200, 2008.
- Kiefer K et al. Canine adipose derived stem cell viability following exposure to synovial fluid from osteoarthritic joints. : Veterinary Orthopaedics Society. 40th Annual Conference Abstracts March, 2013, Utah, USA.
- Vilar J M et al. Assessment of the effect of intraarticular injection of autologous adipose-derived mesenchymal stem cells in osteoarthritic dogs using a double blinded force platform analysis. BMC Veterinary Research. 2014; 10:143.
- Kisiday J, P Kopesky, C Evans, A Grodzinsky, W McIlwraith, D Frisbie. 2007. Evaluation of Adult Equine Bone Marrow- and Adipose-Derived Progenitor Cell Chondrogenesis in Hydrogel Cultures. Wiley InterScience. Vol 26. 322–331.
- Krampera M, G Pizzolo, G Aprili, M Franchini. Mesenchymal stem cells for bone, cartilage, tendon and skeletal muscle repair. Bone. Vol. 39. 678–683.
- Zaidi N, A Nixon. 2007. Stem cell therapy in bone repair and regeneration. Ann N Y Acad Sci. 2007; 1117. 62–72.
Mesenchymal Stem Cells in Aplasia and Bone Marrow Hypoplasia

Bone marrow aplasia or hypoplasia, also known as aplastic anemia (AA) is characterized by peripheral pancytopenia of varying degrees due to spinal cord exhaustion of hematological lines (erythroid, myeloid and or megakaryocytic), in addition to a fatty marrow . In some patients, AA is associated with chemical or drug exposure, or infections with intracellular parasites (viruses, hematozoa, etc.), chronic inflammatory processes and others. In such cases, therapeutic alternatives do not bring a cure, even when the underlying cause is removed. Patients who do not progress to death initially are doomed to blood transfusions and use of medications steadily, until they develop reactions to transfusions and or acquire systemic diseases resulting from the side effects of traditional medications. MSC therapy promotes an extrinsic replacement of mesenchymal stem cells in the bone marrow, provides a stimulus in hematopoiesis, through the production of cytokines and chemokines such as SDF-1, IL-6 and IL-10, colony growth factors G -CSF (granulocyte colony stimulating factor), GM-CSF (macrophage colony stimulating factor) and VEGF (endothelial vascular growth factor), which stimulate the production of sinusoid capillarity, in addition to supporting bone marrow stroma to the modulate differentiation into osteoblasts and adipocytes. These actions allow the bone marrow to become hematopoietically active, with complete and orderly maturation of the hematological lines in a definitive manner.
Read More
- Fouillard, L. et al. Engraftment of allogeneic mesenchymal stem cells in the bone marrow of a patient with severe idiopathic aplastic anemia improves stroma. 2003 Feb;17(2):474-6.
- Jessica de O. L. Castanheira J O L, Wenceslau C V, Barros M A, Gonçalves S, Kerkis I. Not bone marrow-derived mesenchymal stem cells in the treatment of bone marrow disorder in dogs. Rev. Acad., Ciênc. Agrár. Ambient. Curitiba, v. 12, Supl. 1, p. S74, jan./dez. 2014

Mesenchymal Stem Cells in Dry eye syndrome

Dry eye syndrome (DES), also known as keratoconjunctivitis sicca (KCS) is a pathology of high incidence in dogs. Among the various etiologies, autoimmune KCS results in a destruction of the tear glands that leads to a drastic decrease in the production of tears (watery portion normally), causing the eye to become dry, lose its luster and start to produce a thick secretion. Currently, all patients with this diagnosis need daily immunomodulatory medications with high frequency, otherwise the lack of eye lubrication can lead to important corneal lesions leading to loss of the eye. MSCs have shown to be highly effective in this pathology. Through the residual immunomodulatory action, they do on the glands, patients return to normal tears and no longer need conventional medications.
Read More
- Shin AE Park, et al. Safety and immunomodulatory effects of allogeneic canine adipose-derived mesenchymal stromal cells transplanted into the region of the lacrimal gland, the gland of the third eyelid and the knee joint. Cytotherapy, 2013; 15: 1498e1510
- Villatoro A J et al. Use of Adipose-Derived Mesenchymal Stem Cells in Keratoconjunctivitis Sicca in a Canine Model BioMed Research International Volume 2015, Article ID 527926, 10 pages
- Bittencourt M K W, Barros M A Allogeneic Mesenchymal Stem Cell Transplantation in Dogs With Keratoconjunctivitis Sicca. Cell Medicine, Vol. 8, pp. 63–77, 2016.
